FF Schneider
Medical Device Engineering
Medical Device Engineering
Portable airway clearance device for patients with respiratory muscle weakness. Also called cough-assist device.

Basic Safety and Essential Performance
Medical Devices - Quality management systems
under code 80488299002
Medical electrical equipment: Usability
Medical electrical equipment used in the home healthcare environment
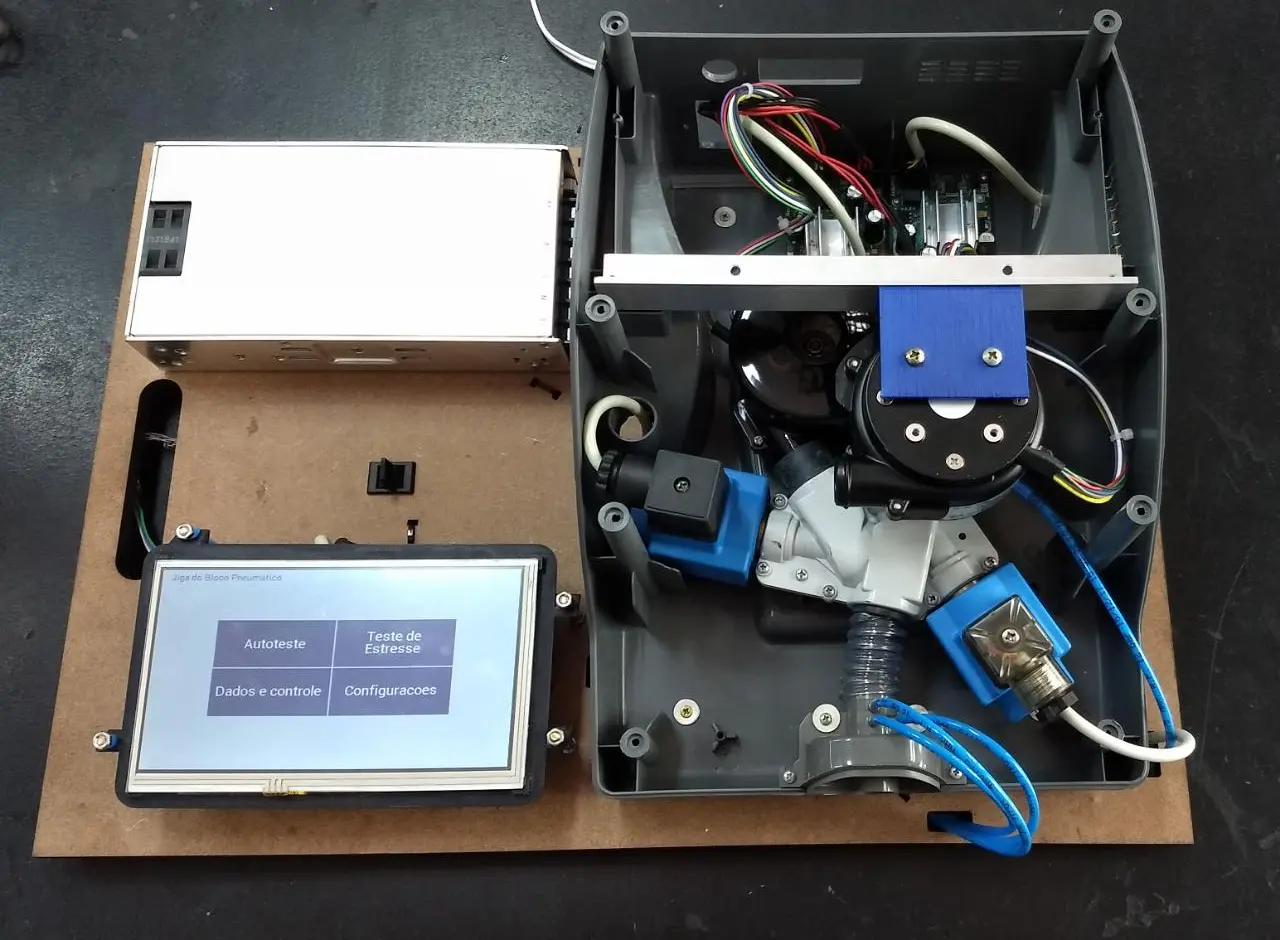
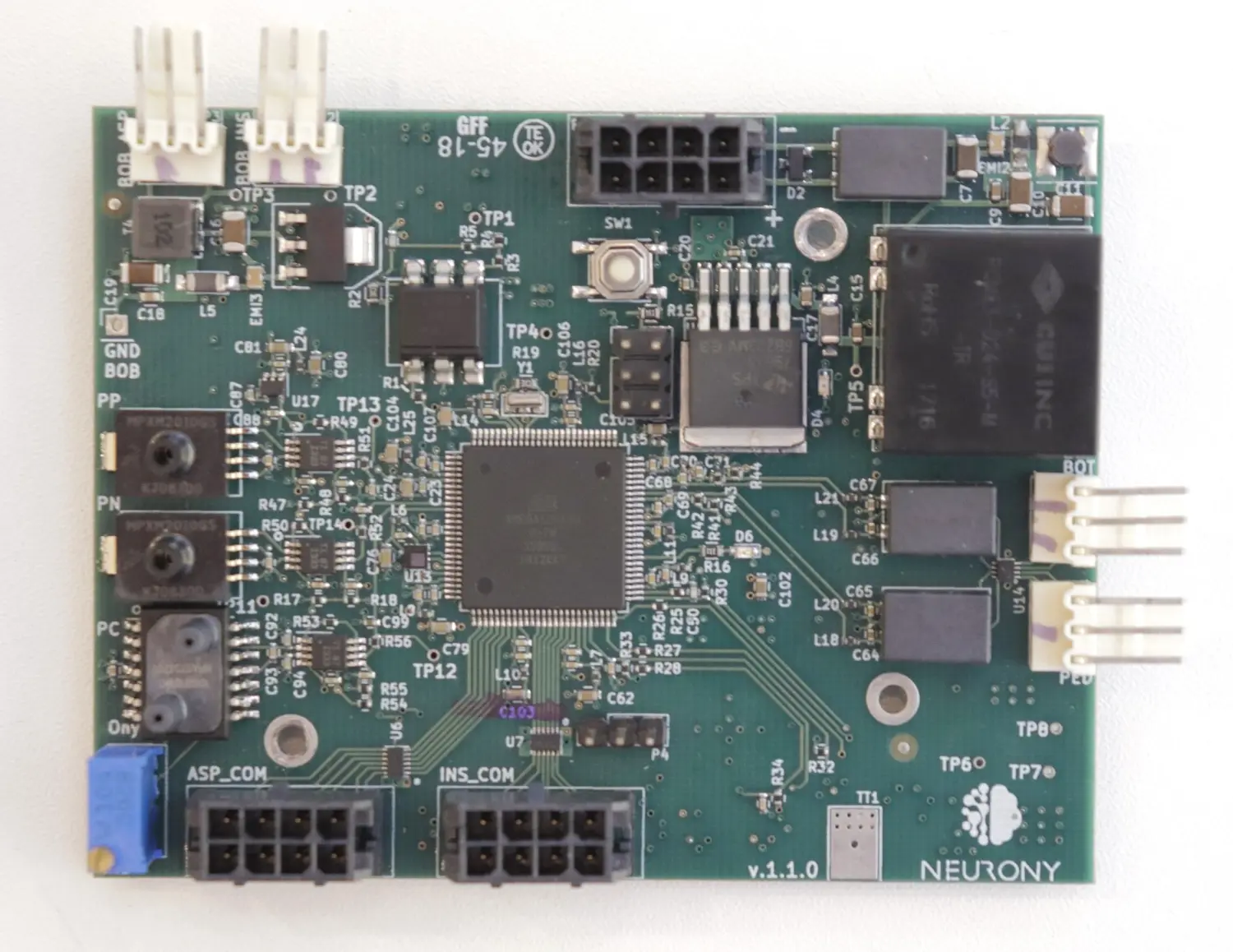
Developed between 2016 and 2018, this project aimed to provide a cost-effective, locally-produced cough assist machine for the Brazilian market.
I worked directly with patients and technical staff, employing Human-Centered Design principles to align the product with medical and user requirements while addressing market needs.
From concept to stable production, the device was completed within two years and is certified by ANVISA under code 80488299002. It remains in the market, with thousands of units sold over the years by a single small manufacturer.







We can develop similar devices or adapt this technology to your specific needs. Let's discuss your project.
Schedule a ConsultationOr send an email to: Loading...