FF Schneider
Medical Device Engineering
Medical Device Engineering
COVID-19 Mechanical ventilator designed for patients not dependent on mechanical ventilation

Basic Safety and Essential Performance
Medical Devices - Quality management systems
COVID-19 fast-track protocol
Medical electrical equipment: Usability
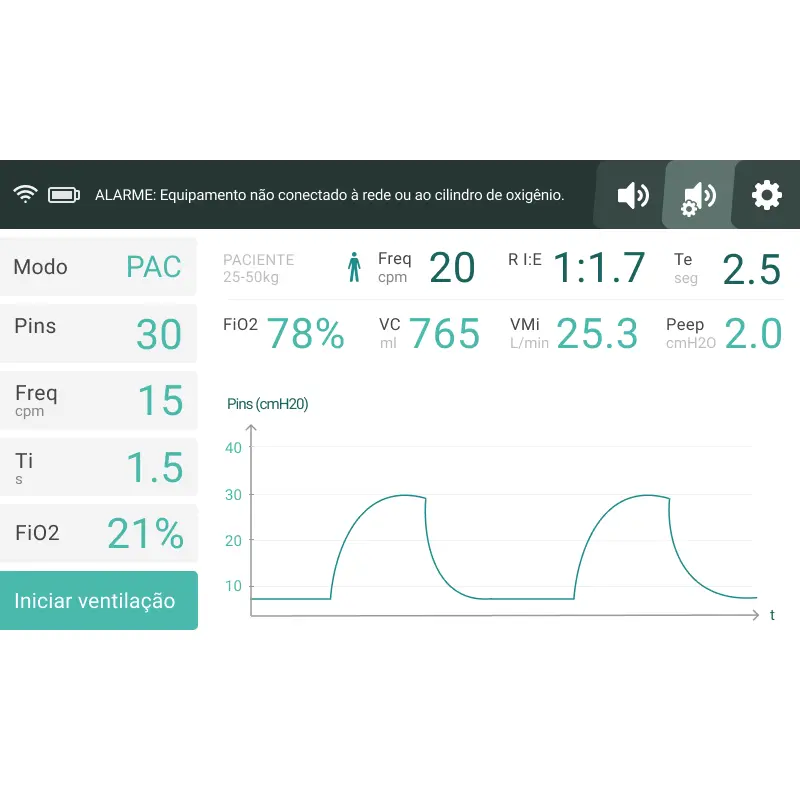
Developed and certified in only 6 months during the critical days of the COVID pandemic, this mechanical ventilator was designed for Brazilian patients not fully dependent on mechanical ventilation for survival, as many researches have shown that non-invasive ventilatory support help increase the survival rates of COVID-19 patients.
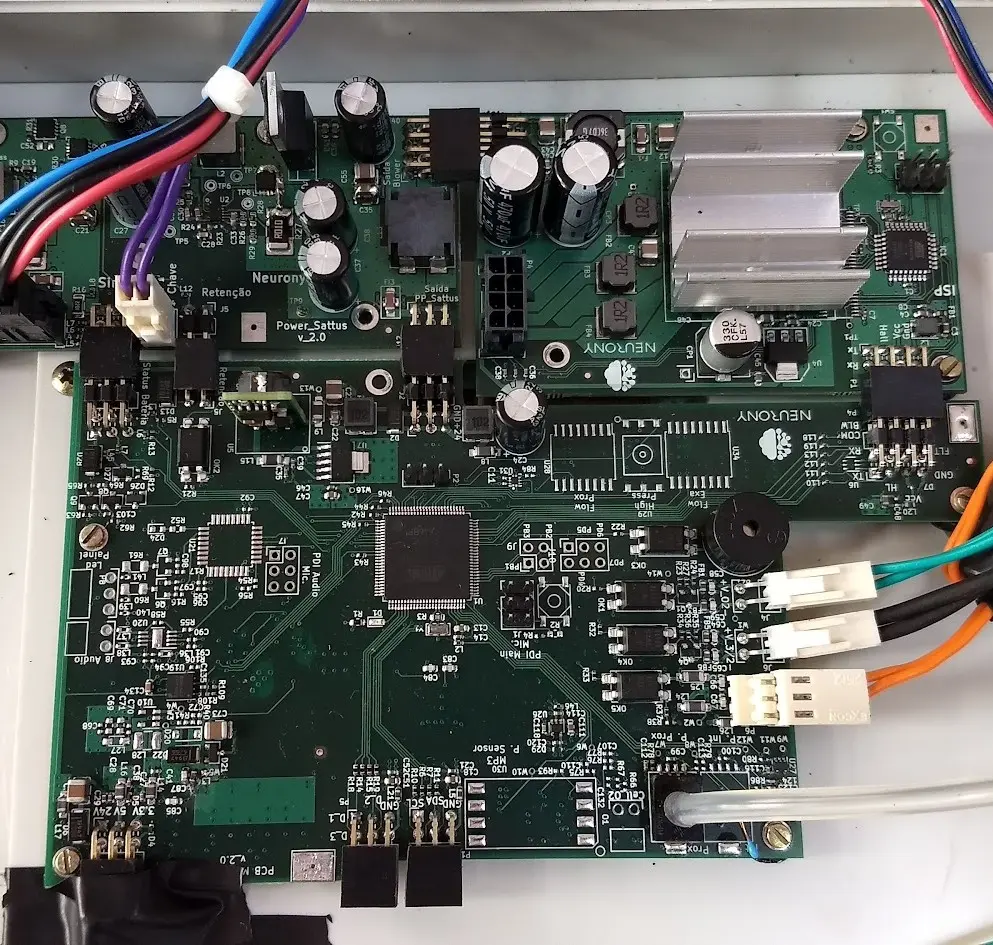
Its development faced huge challenges, including shortages of raw materials, electronic components, and specialized workforce. To overcome these hurdles, our team partnered with Whirlpool engineers, finding innovative solutions.
These hard times provided valuable lessons and strengthened my ability to adapt and deliver under extreme conditions.







We can develop similar devices or adapt this technology to your specific needs. Let's discuss your project.
Schedule a ConsultationOr send an email to: Loading...