FF Schneider
Medical Device Engineering
Medical Device Engineering
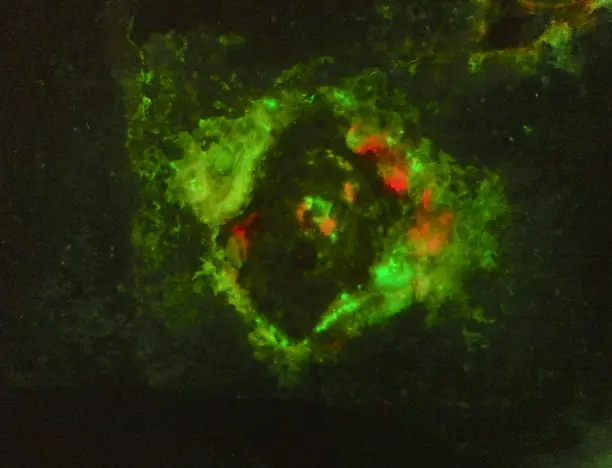
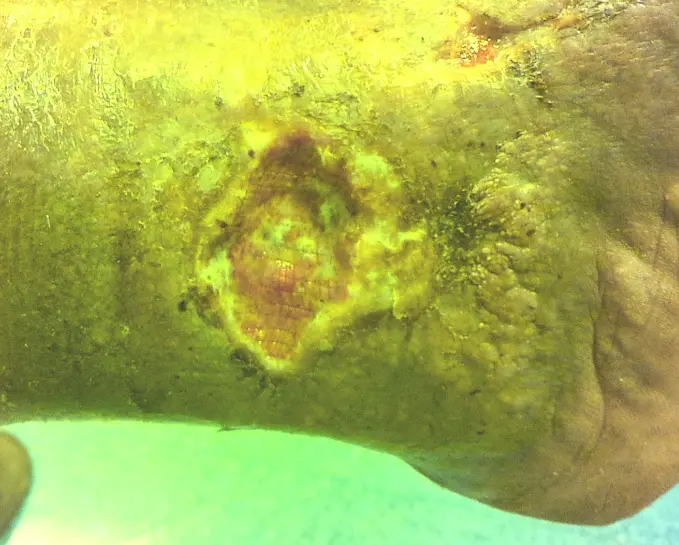
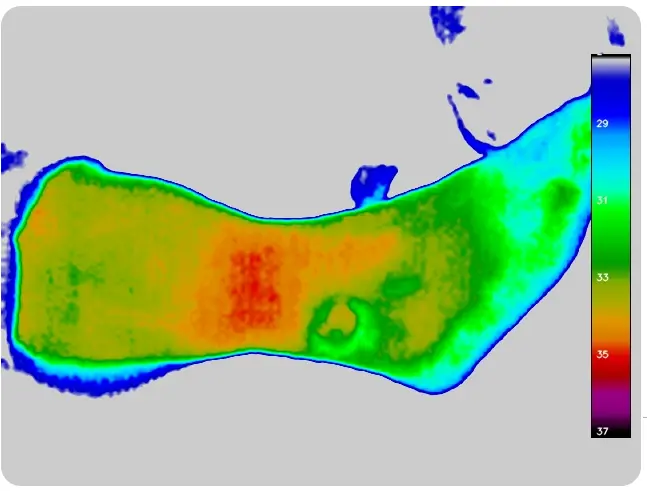
Advanced imaging technology for precise wound measurement, tracking, and bacterial load evaluation

Basic Safety and Essential Performance
Electromagnetic Compatibility
Application of Risk Management to Medical Devices
Medical device software - Software life cycle processes
Photobiological safety of lamps and lamp systems
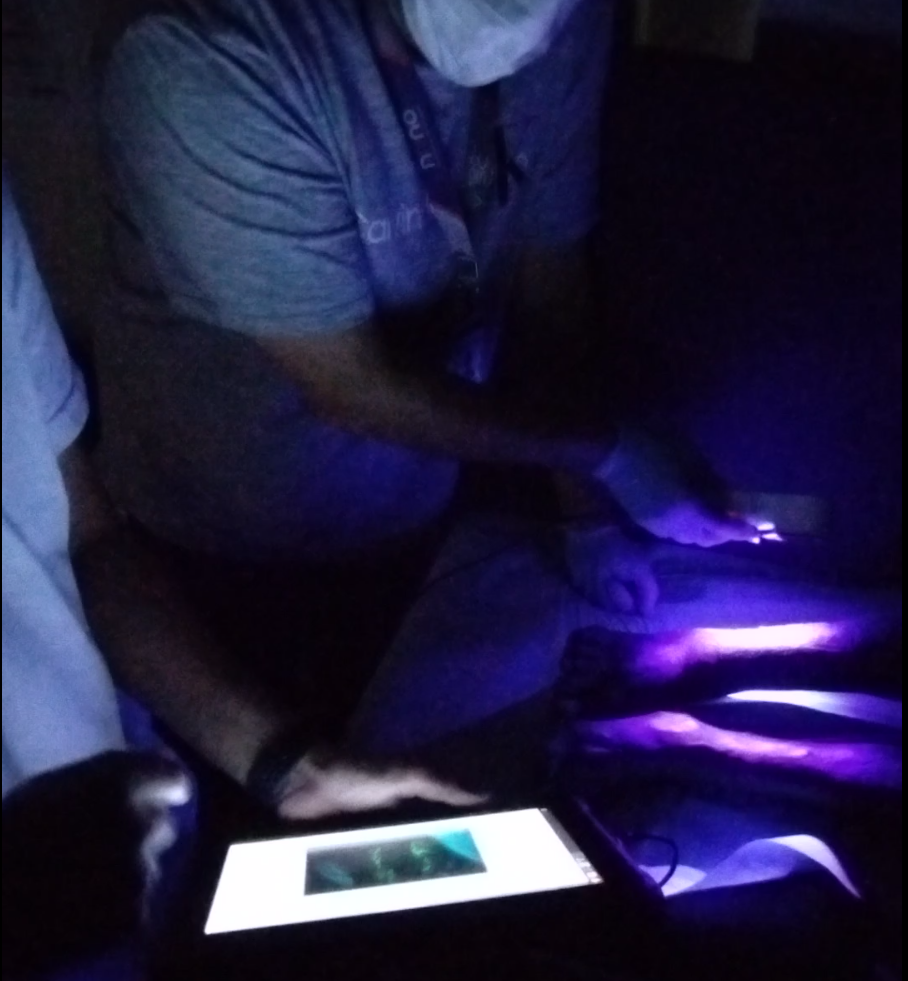
This portable medical device enhances physicians' diagnostic capabilities by extracting key wound parameters invisible to the naked eye. It achieves this through a combination of specialized cameras, light sources, sensors, and AI.
Integrated with an online platform, it has the potential to revolutionize the wound-assessment market, benefiting countless patients.
Developing this device introduced new challenges, including design for manufacturability (DfM) for high production volumes, image processing and refinement, and the development of AI features tailored to medical applications.





We can develop similar devices or adapt this technology to your specific needs. Let's discuss your project.
Schedule a ConsultationOr send an email to: Loading...