FF Schneider
Medical Device Engineering
Medical Device Engineering
Advanced wound therapy system based on violet and IR light with smart monitoring
Basic Safety and Essential Performance
Medical Devices - Quality management systems
Medical device software - Software life cycle processes
Photobiological safety of lamps and lamp systems - Risk Group 2
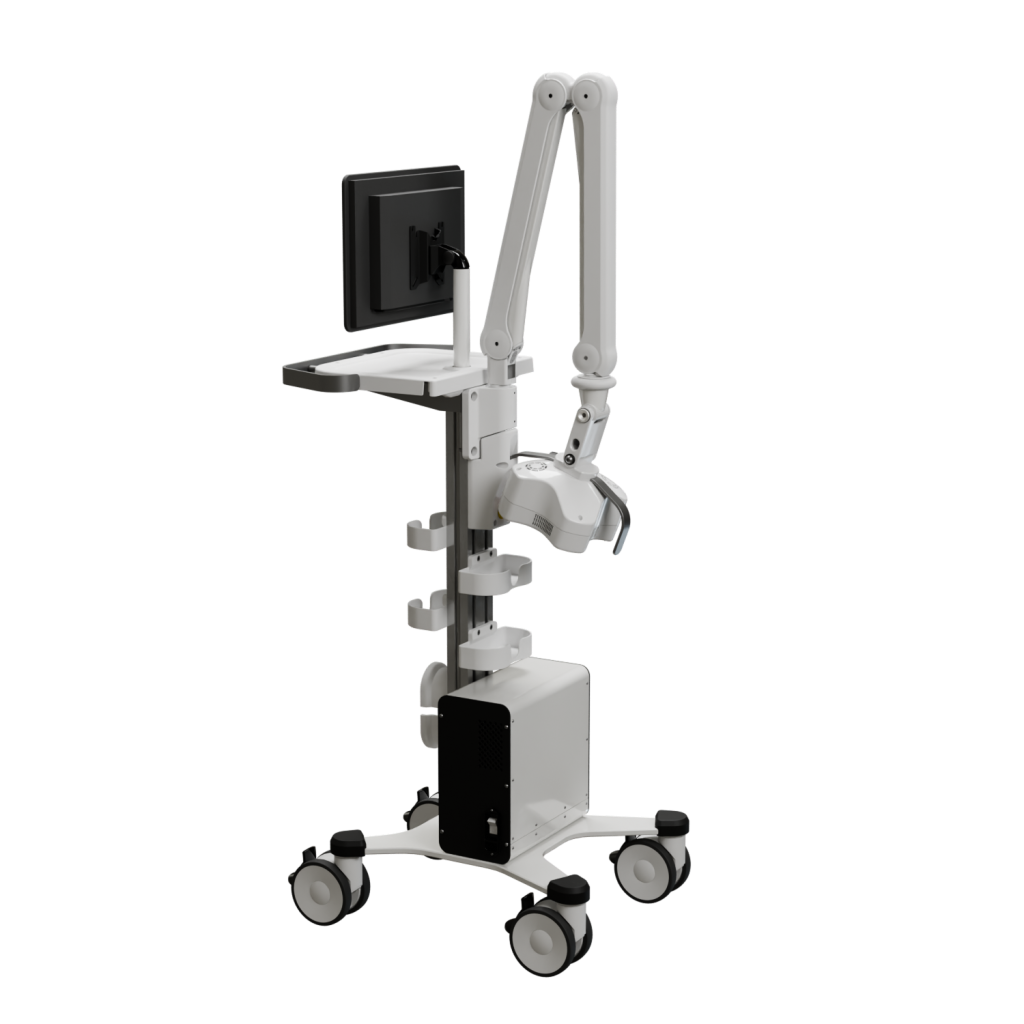
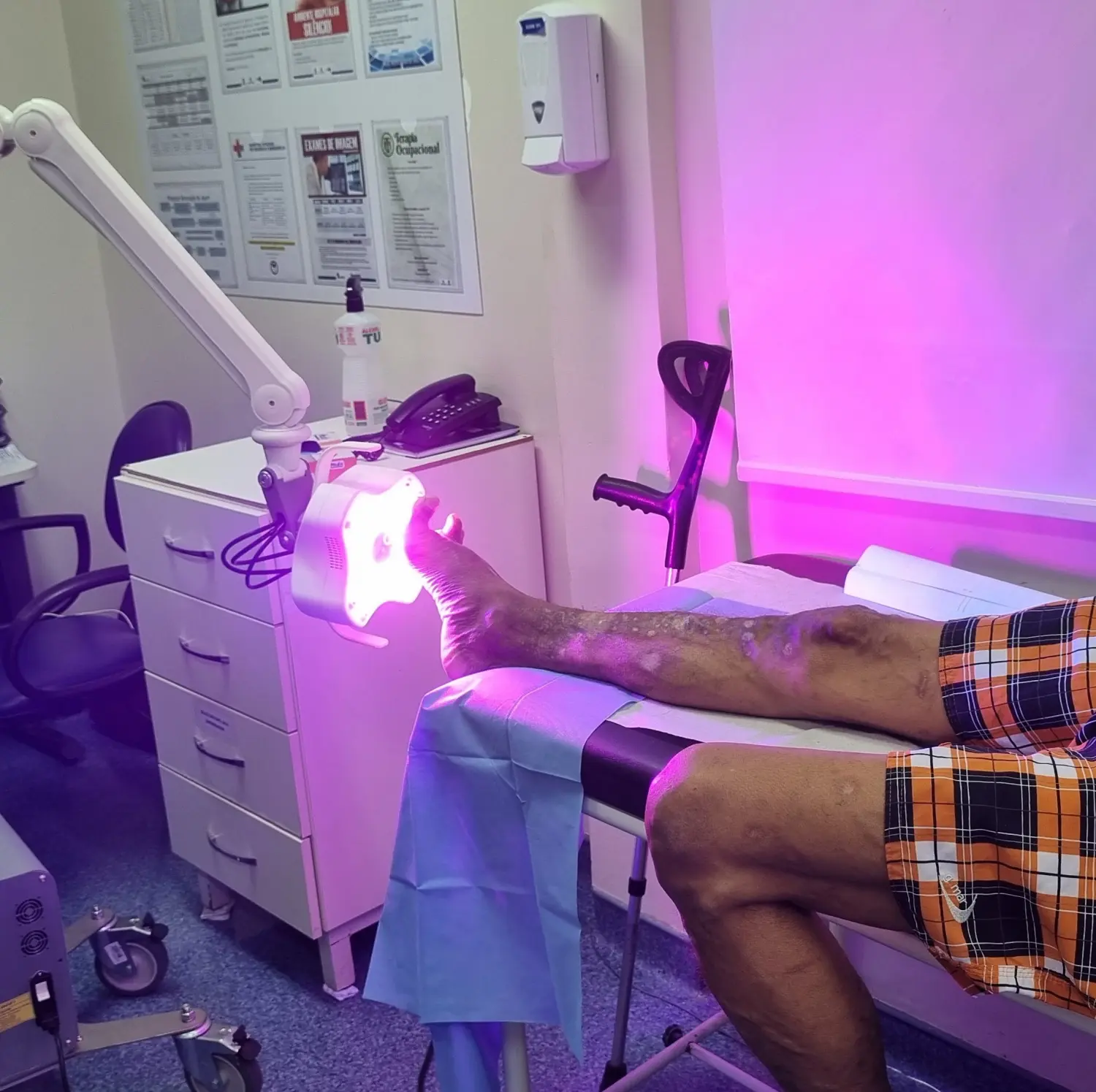
This revolutionary Class II medical device, FDA-approved under K233077, was developed by a multinational team of 10 engineers and medical advisors under my leadership.
The device employs a groundbreaking approach to chronic wound treatment, combining non-coherent light therapy with advanced camera systems to assist operators and ensure patient safety.
This complex system integrates multiple custom and OTS PCBs, supports a variety of communication protocols and programming languages, and features an intuitive user interface designed for seamless operation. Led it from the start to the production.
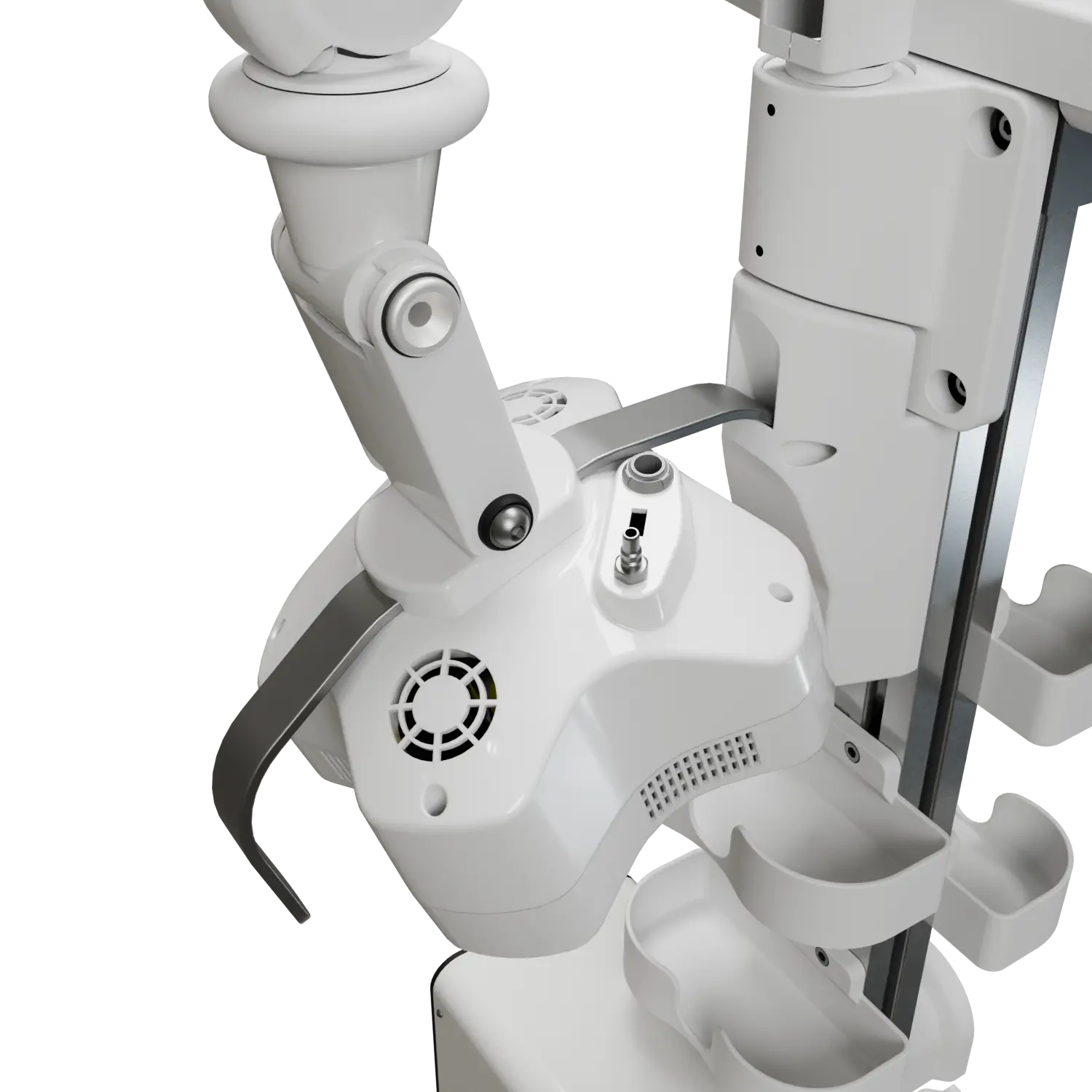
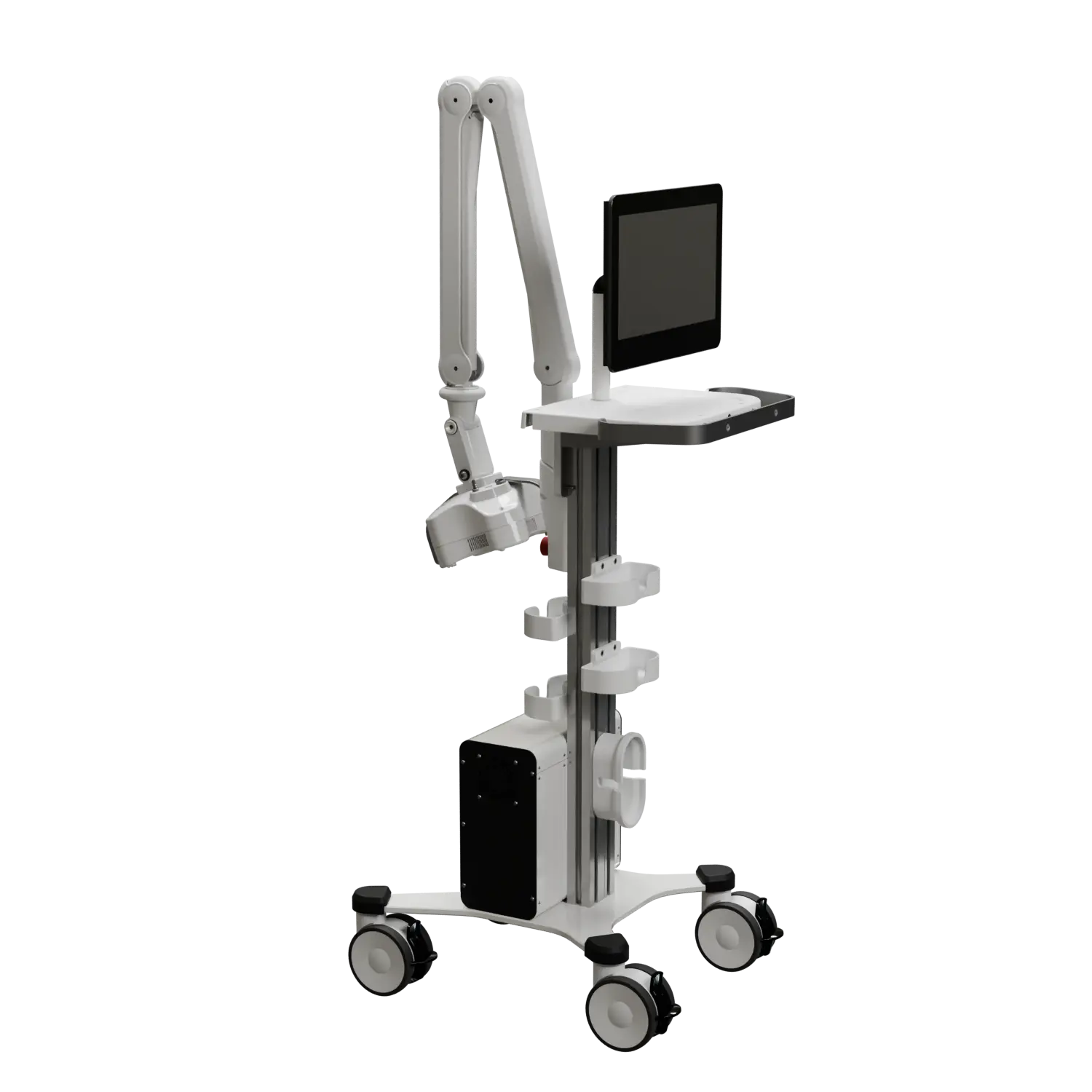




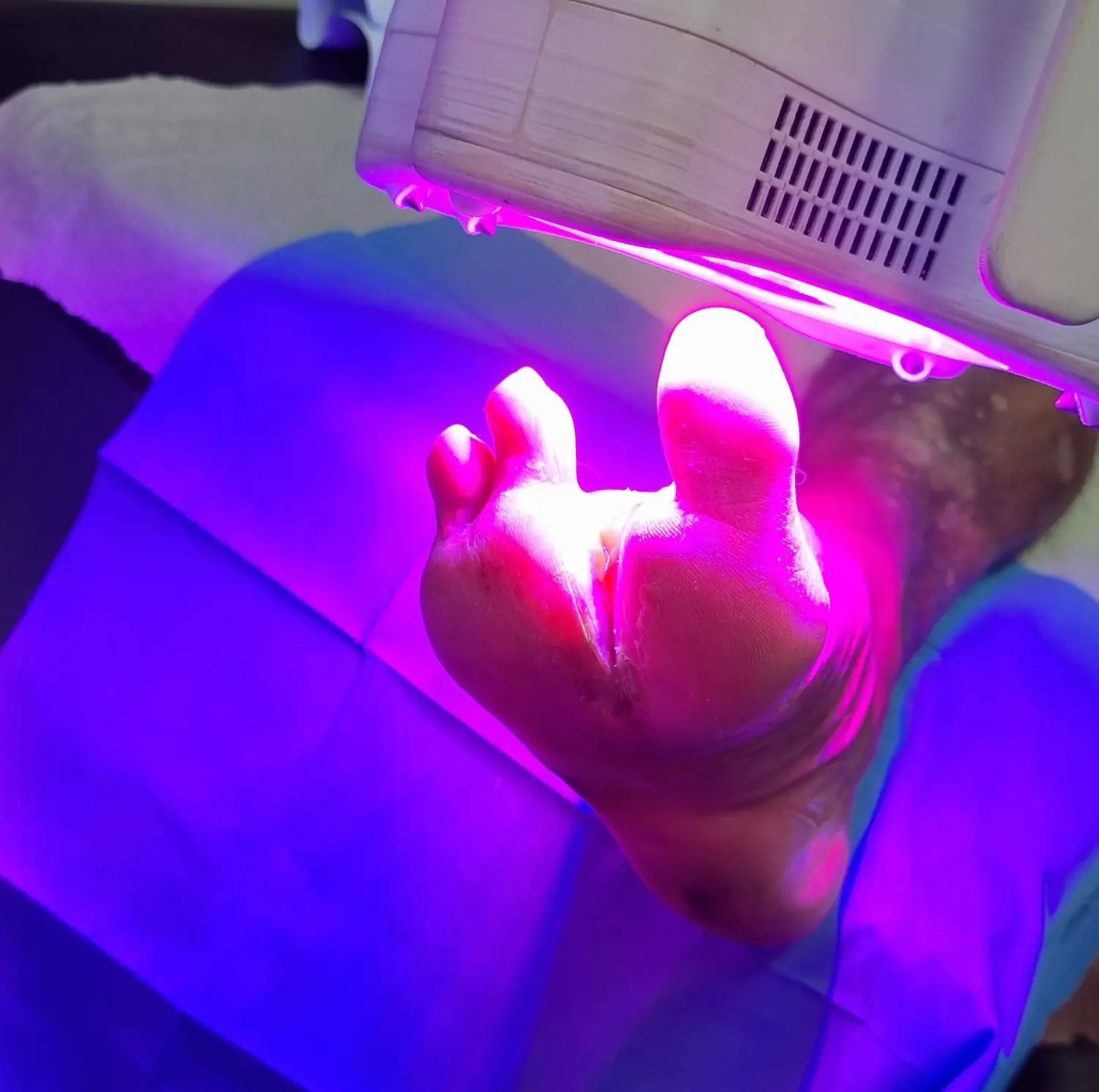

We can develop similar devices or adapt this technology to your specific needs. Let's discuss your project.
Schedule a ConsultationOr send an email to: Loading...