Featured Projects

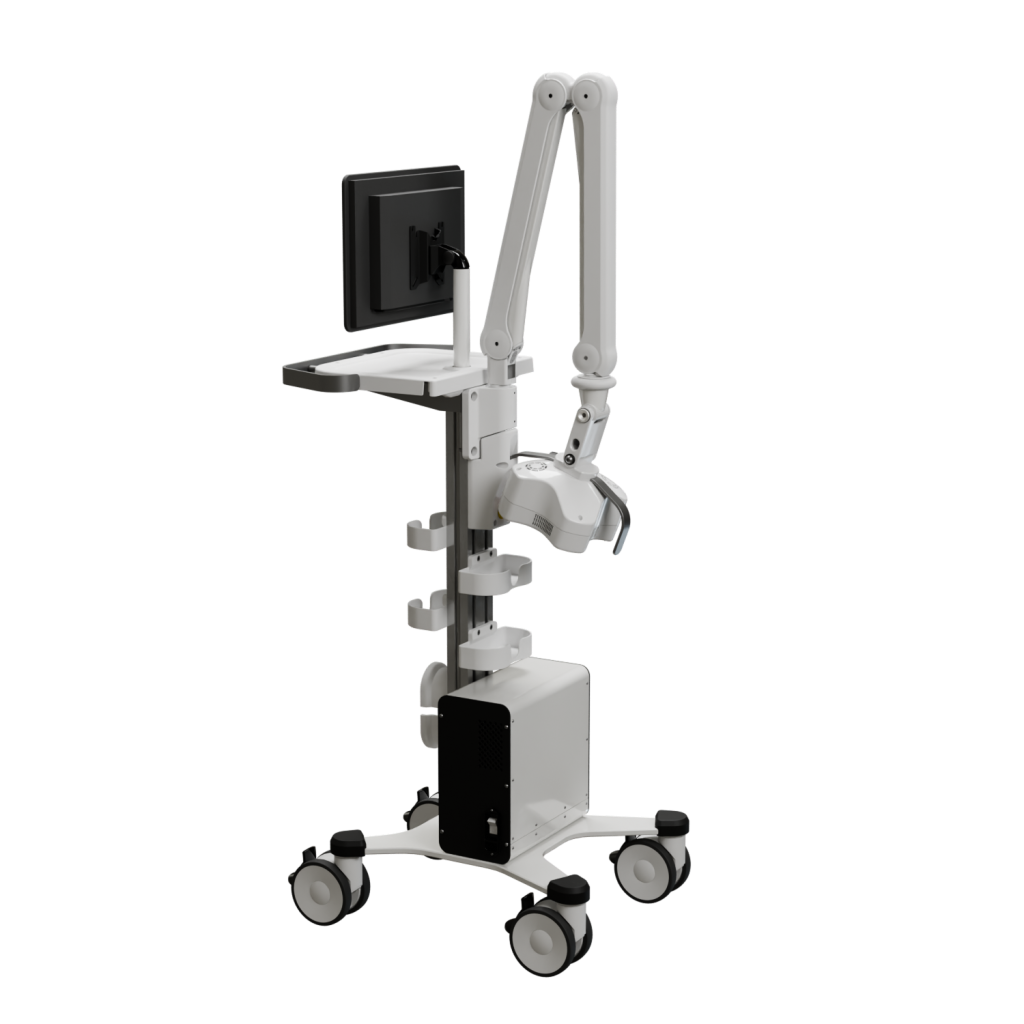


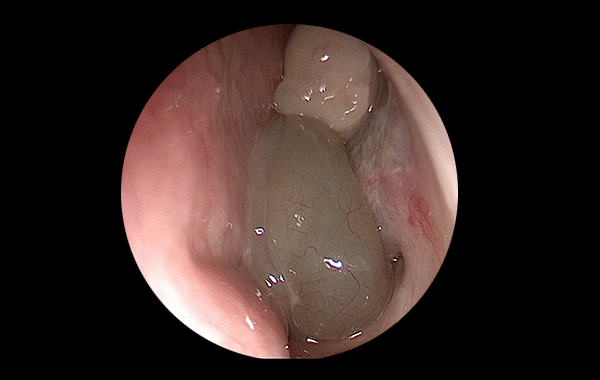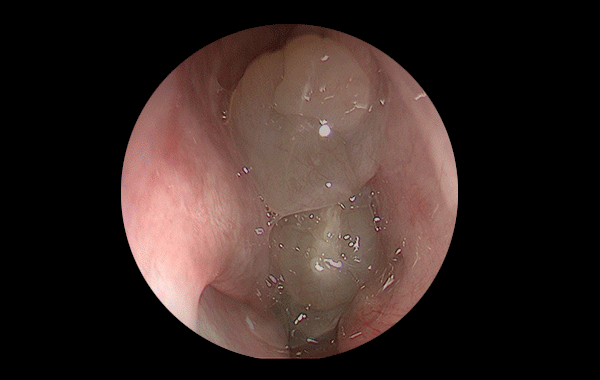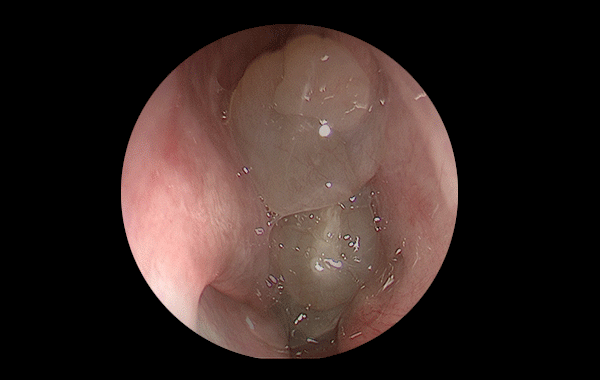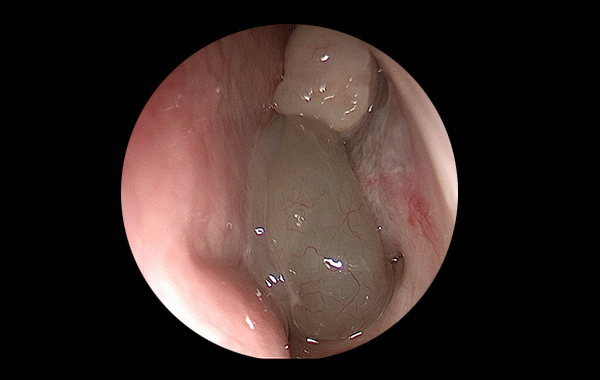


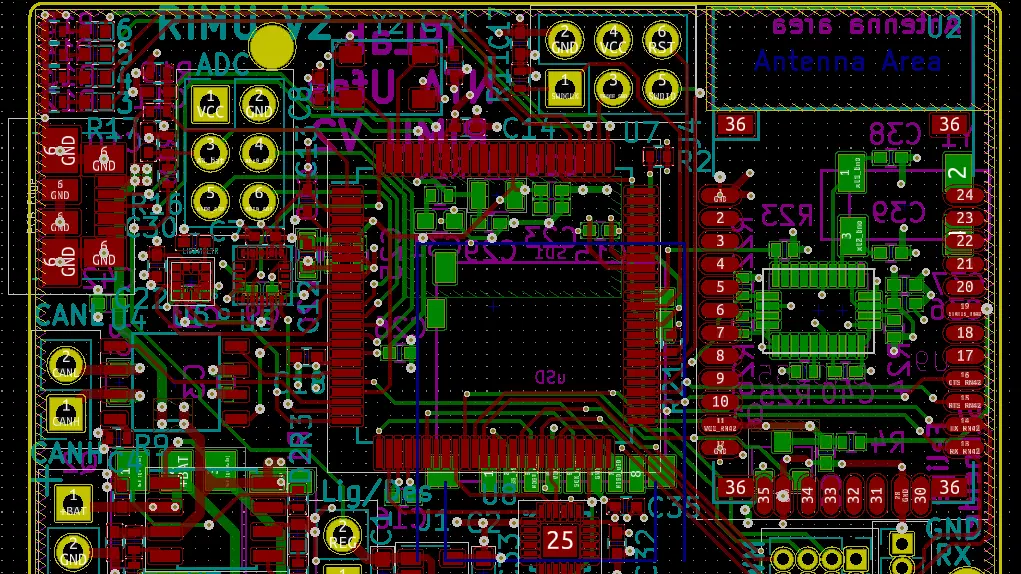
Comprehensive project leadership from concept to final product. Expertise spans chronic wounds, respiratory systems, photobiomodulation, and endoscopy, ensuring complete oversight of the development lifecycle.
Avoid delays and unexpected costs with expert guidance through complex standards. Strategic adjustments to hardware and software minimize failure risks in safety and EMC testing.
Expertise in IEC 60601, ISO 13485, and FDA regulations ensures comprehensive documentation preparation, helping your device not only function properly but successfully reach the market.
Access to ILAC-approved laboratories in Brazil enables testing at costs up to 80% lower than US or EU options while maintaining full validation for MDR and FDA requirements.
Functional prototypes validate hypotheses quickly, leveraging extensive hardware and firmware development experience to create relevant proof-of-concept steps toward commercialization.
Strategic assembly and management of specialist teams ensures optimal solutions delivered within timelines that align with your specific requirements.









Whether it's a new device, a compliance challenge, or the need for a functional prototype, I'm here to help. Let's discuss how we can accelerate your development.
Schedule a ConsultationOr send an email to: Loading...